Methods of Recycling, Properties and Applications of Recycled Thermoplastic Polymers
Abstract
فهرست عناوین
The synthesis approach significantly impacts the properties of such materials and these properties in turn have a significant impact on their applications. Due to the ideal properties of the thermoplastic polymers such as corrosion resistance, low density or user-friendly design, the production of plastics has increased markedly over the last 60 years, becoming more used than aluminum or other metals. Also, recycling is one of the most important actions currently available to reduce these impacts and represents one of the most dynamic areas in the plastics industry today.
۱٫ Introduction
Polymeric materials can be classified as thermosets and thermoplastics. Thermoset polymers refer to the irreversible polymerization and this type of polymer is cured by chemical reaction or heat and becomes infusible and insoluble material. Thermoplastics are made up of linear molecular chains and this polymer softens on heating and hardens when cooled [1,2,3,4,5,6].

Thermoplastic polymers are represented by a large range of plastic materials. There are three types of thermoplastic polymers:
- –
-
Crystalline thermoplastics, usually translucent with molecular chains which present a regular arrangement. Compared to other types, these polymers have more mechanical impact resistance. Examples of this type of polymers are polypropylene (PP), low-density polyethylene (LDPE), and high-density polyethylene (HDPE).
- –
-
Amorphous thermoplastics, usually transparent with the molecules arranged randomly. Examples of this type of polymers are poly vinyl chloride (PVC), polymethylmethacrylate (PMMA), polycarbonate (PC), polystyrene (PS), and acrylonitrilebutadiene styrene (ABS).
- –
۲٫ Recycled Thermoplastic Polymers
Also, the plastic manufacturing cost is lower due to its simple mass production (Figure 1). The main reasons which make the thermoplastic polymers used in various applications are:
- –
-
The thermoplastic polymers can be processed by several methods leading to various kinds of plastic products;
- –
-
They are used for a specific application several compounding, operating condition, additives, fillers, and reinforcements;
- –
۲٫۱٫ Primary Recycling
۲٫۲٫ Secondary Recycling or Mechanical Recycling
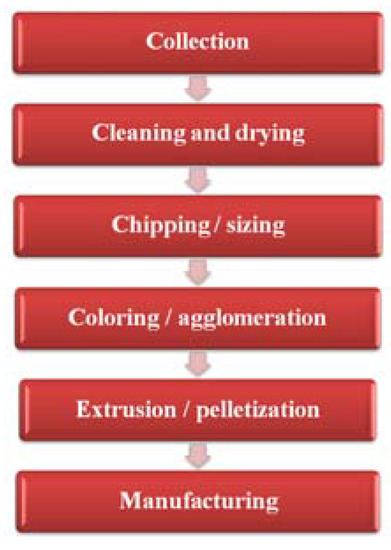
۲٫۳٫ Feedstock or Chemical Recycling
The chemical reactions used for decomposition of polymers into monomers are:
- –
-
Hydrogenation
- –
-
Glycolysis
- –
-
Gasification
- –
-
Hydrolysis
- –
-
Pyrolysis
- –
-
Methanolysis
- –
-
Chemical depolymerization
- –
-
Thermal cracking
- –
-
Catalytic cracking and reforming
- –
-
Photodegradation
- –
-
Ultrasound degradation
- –
-
Degradation in microwave reactor.
Hydrolysis is a recycling method that involves a reaction of PET with water in an acid, alkaline or neutral environment, leading to total depolymerization into its monomers. The disadvantages of the hydrolysis method are represented by the high temperatures (between 200 and 250 °C), by pressures (between 1.4 and 2 MPa) and by the long time needed to complete depolymerization. This method is not widely used, because of the high cost. Hydrolysis of PET flakes can be carried out by:
- –
-
Alkaline hydrolysis, which uses an aqueous alkaline solution of NaOH or KOH, of a concentration of 4–۲۰ wt %. In a study, PET from post-consumer soft-drink bottles was cut into small pieces and then subjected to alkaline hydrolysis. After that, an autoclave was used; the temperature ranged from 120–۲۰۰ °C with aqueous NaOH solutions and at 110–۱۲۰ °C with a nonaqueous solution of KOH in methyl cellosolve. Sulfuric acid was used to separate terephthalic acid (TPA) of high purity. It was reported that it was obtained about 2% admixture of isophthalic acid together with the pure 98% TPA and the activation energy calculated was 99 kJ/mol [42]. Also, it was demonstrated by alkaline hydrolysis the possibility of simultaneously recycling PET and PVC from PVC-coated woven fabrics [43].
- –
-
Acid hydrolysis used concentrated sulfuric acid and also other mineral acids like nitric or phosphoric acid. In several studies, it was reported that the depolymerization of PET powder from waste bottles using nitric acid was carried out at temperatures between 70 and 100 °C [44,45]. Also, it was reported the hydrolysis of PET using sulfuric acid (96 wt %) at room temperature (30 °C) [46].
- –
-
Neutral hydrolysis, using hot water or steam, high-pressure autoclaves at temperatures between 200 and 300 °C and pressures of 1–۴ MPa. It was reported that this method is more effective at temperatures higher than 245 °C, with complete depolymerization taking place at 275 °C and its TPA yield being usually above 95% [42].
۲٫۴٫ Energy Recovery or Quaternary Recycling

۳٫ Applications of Recycled Thermoplastic Polymers

۳٫۱٫ Recycled Polymers for Food Industry
The big problem with these recycled polymers refers to the possibility of contamination of virgin material and from contamination during the previous use of packaging and during production processes. The contamination of these materials may be of a chemical or microbiological nature [16]. Also, the Food and Drug Administration (FDA) has announced that their concerns with the use of recycled plastic materials in food-contact are related to:
- –
-
contaminants found in the post-consumer material which may appear in the final food-contact product made from the recycled material;
- –
-
recycled post-consumer material not suitable for food-contact use may be incorporated into food-contact packaging;
- –
-
the adjuvants from the recycled plastic may not respect the regulations for food-contact use [69].
For example, the reused plastics can be contaminated by pollutants and the pollutants may migrate into the packaged goods in the next life of the material. The following methods are recommended to avoid migration of potential contamination:
- –
-
Packaging washing (only if it is used in the same way as before);
- –
-
Plastic depolymerization (the monomers are purified efficiently and the produced polymer is as pure as those made from conventional monomers, but this method is expensive);
- –
-
Using materials which consist of a few layers; the first layer made from a recycled plastic, the second layer that comes into contact with the product is made from virgin material (act as a functional barrier because it is reducing the migration of the contaminants from the recycled plastic into the packaged food). An example of this method is PET; the removing of all contamination from bottles is expensive, so to obtain a plastic with purity required for products which have direct contact with foods is very hard and for this reason PET is used as the outer layer in multilayered containers with the inner layer (virgin material) acting as a functional barrier [16].
۳٫۲٫ Recycled Polymers for Indoor Applications
۴٫ Perspectives for a Green Bio-Industry
۵٫ Conclusions
Conflicts of Interest
References
- Amin, S.; Amin, M. Thermoplastic elastomeric (TPE) materials and their use in outdoor electrical insulation. Rev. Adv. Mater. Sci. ۲۰۱۱, ۲۹, ۱۵–۳۰٫ [Google Scholar]
- Ribeiro, M.C.S.; Fiúza, A.; Ferreira, A.; Dinis, M.D.L.; Meira Castro, A.C.; Meixedo, J.P.; Alvim, M.R. Recycling Approach towards Sustainability Advance of Composite Materials’ Industry. Recycling ۲۰۱۶, ۱, ۱۷۸٫ [Google Scholar] [CrossRef]
- Ashori, A. Wood-plastic composites as promising green-composites for automotive industries! Bioresour. Technol. ۲۰۰۸, ۹۹, ۴۶۶۱–۴۶۶۷٫ [Google Scholar] [CrossRef] [PubMed]
- Pascault, J.-P.; Sautereau, H.; Verdu, J.; Williams, R.J. Thermosetting Polymers; CRC Press: Roca Raton, FL, USA, 2002; Volume 64. [Google Scholar]
- Brazel, C.S.; Rosen, S.L. Fundamental Principles of Polymeric Materials; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Yang, Y.; Boom, R.; Irion, B.; van Heerden, D.-J.; Kuiper, P.; de Wit, H. Recycling of composite materials. Chem. Eng. Process. Process Intensif. ۲۰۱۲, ۵۱, ۵۳–۶۸٫ [Google Scholar] [CrossRef]
- Schlosser, E.; Nass, B.; Wanzke, W. Flame Retardant Combination for Thermoplastic Polymers L. U.S. Patent 6,547,992, 15 April 2003. [Google Scholar]
- Bicerano, J. Prediction of Polymer Properties; CRC Press: Roca Raton, FL, USA, 2002. [Google Scholar]
- Ultem, L.; STM, U.S.; Ultrablend, S. Polymer Science Dictionary; Springer: Dordrecht, The Netherlands, 2017; ISBN 978-94-024-0893-5. [Google Scholar]
- Nicholson, J. The Chemistry of Polymers; Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Giboz, J.; Copponnex, T.; Mélé, P. Microinjection molding of thermoplastic polymers: A review. J. Micromech. Microeng. ۲۰۰۷, ۱۷, R96. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Khan, M.I.; Zagho, M.; Shakoor, R. A Brief Overview of Shape Memory Effect in Thermoplastic Polymers. In Smart Polymer Nanocomposites; Springer: Berlin, Germany, 2017; pp. 281–۳۰۱٫ [Google Scholar]
- Wei, C.; Esposito, D.; Tauer, K. Thermal properties of thermoplastic polymers: Influence of polymer structure and procedure of radical polymerization. Polym. Degrad. Stab. ۲۰۱۶, ۱۳۱, ۱۵۷–۱۶۸٫ [Google Scholar] [CrossRef]
- Michler, G.H.; Balta-Calleja, F.J. Mechanical Properties of Polymers Based on Nanostructure and Morphology; CRC Press: Roca Raton, FL, USA, 2016; Volume 71. [Google Scholar]
- Kolek, Z. Recycled polymers from food packaging in relation to environmental protection. Pol. J. Environ. Stud. ۲۰۰۱, ۱۰, ۷۳–۷۶٫ [Google Scholar]
- Jiun, Y.L.; Tze, C.T.; Moosa, U.; Mou’ad, A.T. Effects of Recycling Cycle on Used Thermoplastic Polymer and Thermoplastic Elastomer Polymer. Polym. Polym. Compos. ۲۰۱۶, ۲۴, ۷۳۵٫ [Google Scholar]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. ۲۰۰۹, ۳۶۴, ۲۱۱۵–۲۱۲۶٫ [Google Scholar] [CrossRef] [PubMed]
- Drobny, J.G. Handbook of Thermoplastic Elastomers; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Rodriguez, F.; Cohen, C.; Ober, C.K.; Archer, L. Principles of Polymer Systems; CRC Press: Roca Raton, FL, USA, 2014. [Google Scholar]
- Lee, S.-T.; Park, C.B. Foam Extrusion: Principles and Practice; CRC Press: Roca Raton, FL, USA, 2014. [Google Scholar]
- Fried, J.R. Polymer Science and Technology; Pearson Education: London, UK, 2014. [Google Scholar]
- Myshkin, N.; Pesetskii, S.; Grigoriev, A.Y. Polymer Tribology: Current State and Applications. Tribol. Ind. ۲۰۱۵, ۳۷, ۲۸۴–۲۹۰٫ [Google Scholar]
- Park, H.M.; Li, X.; Jin, C.Z.; Park, C.Y.; Cho, W.J.; Ha, C.S. Preparation and properties of biodegradable thermoplastic starch/clay hybrids. Macromol. Mater. Eng. ۲۰۰۲, ۲۸۷, ۵۵۳–۵۵۸٫ [Google Scholar] [CrossRef]
- Koo, J.H. Polymer Nanocomposites: Processing, Characterization, and Applications; McGraw-Hill Professional Pub.: New York, NY, USA, 2006. [Google Scholar]
- Schulz, U. Review of modern techniques to generate antireflective properties on thermoplastic polymers. Appl. Opt. ۲۰۰۶, ۴۵, ۱۶۰۸–۱۶۱۸٫ [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.K.; Hwang, K.-J.; Ha, C.-S. Preparation, mechanical and rheological properties of a thermoplastic polyolefin (TPO)/organoclay nanocomposite with reference to the effect of maleic anhydride modified polypropylene as a compatibilizer. Polymer ۲۰۰۵, ۴۶, ۱۹۹۵–۲۰۰۲٫ [Google Scholar] [CrossRef]
- Turi, E. Thermal Characterization of Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Mano, J.; Koniarova, D.; Reis, R. Thermal properties of thermoplastic starch/synthetic polymer blends with potential biomedical applicability. J. Mater. Sci. Mater. Med. ۲۰۰۳, ۱۴, ۱۲۷–۱۳۵٫ [Google Scholar] [CrossRef] [PubMed]
- Wollerdorfer, M.; Bader, H. Influence of natural fibres on the mechanical properties of biodegradable polymers. Ind. Crops Prod. ۱۹۹۸, ۸, ۱۰۵–۱۱۲٫ [Google Scholar] [CrossRef]
- Nichols, C.; Moore, T.; Griffith, S. Thermoplastic Polymers with Improved Infrared Reheat Properties. U.S. Patent 09/973,436, 9 October 2001. [Google Scholar]
- Van de Velde, K.; Kiekens, P. Thermoplastic polymers: Overview of several properties and their consequences in flax fibre reinforced composites. Polym. Test. ۲۰۰۱, ۲۰, ۸۸۵–۸۹۳٫ [Google Scholar] [CrossRef]
- Mohammadzadeh, M. Characterization of Recycled Thermoplastic Polymers. Master’s Thesis, University of Borås, Borås, Sweden, 2009. [Google Scholar]
- Saleh, T.A.; Danmaliki, G.I. Polymer Consumption, Environmental Concerns, Possible Disposal Options, and Recycling for Water Treatment. In Advanced Nanomaterials for Water Engineering, Treatment, and Hydraulics; IGI Global: Hershey, PA, USA, 2017; pp. 200–۲۲۲٫ [Google Scholar]
- Favis, B.D.; Le Corroller, P. Polymeric material and process for recycling plastic blends. U.S. Patent 9,670,344, 6 July 2017. [Google Scholar]
- Francis, R. Recycling of Polymers: Methods, Characterization and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. ۲۰۱۷, ۱۱۵, ۴۰۹–۴۲۲٫ [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.S. Chemical recycling of carbon dioxide to methanol and dimethyl ether: From greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J. Org. Chem. ۲۰۰۸, ۷۴, ۴۸۷–۴۹۸٫ [Google Scholar] [CrossRef] [PubMed]
- Achilias, D.S.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, Ε.V. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. ۲۰۰۷, ۱۴۹, ۵۳۶–۵۴۲٫ [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S. Enzyme-Catalyzed Synthesis and Chemical Recycling of Polyesters. Macromol. Biosci. ۲۰۰۲, ۲, ۱۰۵–۱۲۶٫ [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Achilias, D.S. Chemical Recycling of Poly(ethylene terephthalate). Macromol. Mater. Eng. ۲۰۰۷, ۲۹۲, ۱۲۸–۱۴۶٫ [Google Scholar] [CrossRef]
- Karayannidis, G.; Chatziavgoustis, A.; Achilias, D. Poly (ethylene terephthalate) recycling and recovery of pure terephthalic acid by alkaline hydrolysis. Adv. Polym. Technol. ۲۰۰۲, ۲۱, ۲۵۰–۲۵۹٫ [Google Scholar] [CrossRef]
- Kumagai, S.; Hirahashi, S.; Grause, G.; Kameda, T.; Toyoda, H.; Yoshioka, T. Alkaline hydrolysis of PVC-coated PET fibers for simultaneous recycling of PET and PVC. J. Mater. Cycles Waste Manag. ۲۰۱۷, ۱–۱۱٫ [Google Scholar] [CrossRef]
- Yoshioka, T.; Motoki, T.; Okuwaki, A. Kinetics of hydrolysis of poly (ethylene terephthalate) powder in sulfuric acid by a modified shrinking-core model. Ind. Eng. Chem. Res. ۲۰۰۱, ۴۰, ۷۵–۷۹٫ [Google Scholar] [CrossRef]
- Mehrabzadeh, M.; Shodjaei, S.; Khosravi, M. Chemical recycling of polyethylene terephthalate. Iran. Polym. J. ۲۰۰۰, ۹, ۳۷–۴۰٫ [Google Scholar]
- De Carvalho, G.M.; Muniz, E.C.; Rubira, A.F. Hydrolysis of post-consume poly (ethylene terephthalate) with sulfuric acid and product characterization by WAXD, 13 C-NMR and DSC. Polym. Degrad. Stab. ۲۰۰۶, ۹۱, ۱۳۲۶–۱۳۳۲٫ [Google Scholar] [CrossRef]
- Kosmidis, V.A.; Achilias, D.S.; Karayannidis, G.P. Poly (ethylene terephthalate) recycling and recovery of pure terephthalic acid. Kinetics of a phase transfer catalyzed alkaline hydrolysis. Macromol. Mater. Eng. ۲۰۰۱, ۲۸۶, ۶۴۰–۶۴۷٫ [Google Scholar] [CrossRef]
- Colomines, G.; Robin, J.-J.; Tersac, G. Study of the glycolysis of PET by oligoesters. Polymer ۲۰۰۵, ۴۶, ۳۲۳۰–۳۲۴۷٫ [Google Scholar] [CrossRef]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Flores-Giraldo, L.; Gutiérrez-Ortiz, J.I. Kinetics of catalytic glycolysis of PET wastes with sodium carbonate. Chem. Eng. J. ۲۰۱۱, ۱۶۸, ۳۱۲–۳۲۰٫ [Google Scholar] [CrossRef]
- Yue, Q.F.; Wang, C.X.; Zhang, L.N.; Ni, Y.; Jin, Y.X. Glycolysis of poly(ethylene terephthalate) (PET) using basic ionic liquids as catalysts. Polym. Degrad. Stab. ۲۰۱۱, ۹۶, ۳۹۹–۴۰۳٫ [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, Z.; Zhang, X.; Zhang, S.; Zhang, Y. Glycolysis of poly(ethylene terephthalate) catalyzed by ionic liquids. Eur. Polym. J. ۲۰۰۹, ۴۵, ۱۵۳۵–۱۵۴۴٫ [Google Scholar] [CrossRef]
- Pardal, F.; Tersac, G. Comparative reactivity of glycols in PET glycolysis. Polym. Degrad. Stab. ۲۰۰۶, ۹۱, ۲۵۶۷–۲۵۷۸٫ [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Xiang, H.; Xu, Y.; Li, Y. Study on methanolytic depolymerization of PET with supercritical methanol for chemical recycling. Polym. Degrad. Stab.2002, ۷۵, ۱۸۵–۱۹۱٫ [Google Scholar] [CrossRef]
- Achilias, D.S.; Karayannidis, G.P. The Chemical Recycling of PET in the Framework of Sustainable Development. Water Air Soil Pollut. Focus ۲۰۰۴, ۴, ۳۸۵–۳۹۶٫ [Google Scholar] [CrossRef]
- Kurokawa, H.; Ohshima, M.; Sugiyama, K.; Miura, H. Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Polym. Degrad. Stab. ۲۰۰۳, ۷۹, ۵۲۹–۵۳۳٫ [Google Scholar] [CrossRef]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. ۲۰۱۷, ۱. [Google Scholar] [CrossRef]
- Achilias, D.; Antonakou, E.; Roupakias, C.; Megalokonomos, P.; Lappas, A. Recycling techniques of polyolefins from plastic wastes. Glob. NEST J. ۲۰۰۸, ۱۰, ۱۱۴–۱۲۲٫ [Google Scholar]
- Ke, H.; Li-Hua, T.; Zi-Bin, Z.; Cheng-fang, Z. Reaction mechanism of styrene monomer recovery from waste polystyrene by supercritical solvents. Polym. Degrad. Stab. ۲۰۰۵, ۸۹, ۳۱۲–۳۱۶٫ [Google Scholar] [CrossRef]
- Al-Salem, S.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. ۲۰۰۹, ۲۹, ۲۶۲۵–۲۶۴۳٫ [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.M.; Mark, F.E.; Kingsbury, T.; Vehlow, J.; Yamawaki, T. Energy Recovery in the Sustainable Recycling of Plastics from End-of-Life Electrical and Electronic Products. In Proceedings of the 2005 IEEE International Symposium on Electronics and the Environment, New Orleans, LA, USA, 16–۱۹ May 2005; pp. 83–۹۲٫ [Google Scholar]
- Andrady, A.L. Plastics and the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. ۲۰۱۷, ۶۹, ۲۴–۵۸٫ [Google Scholar] [CrossRef] [PubMed]
- European Association of Plastics Recycling and Recovery. Available online: http://www.epro-plasticsrecycling.org/pages/75/epro_statistics (accessed on 24 June 2017).
- Siddique, R.; Khatib, J.; Kaur, I. Use of recycled plastic in concrete: A review. Waste Manag. ۲۰۰۸, ۲۸, ۱۸۳۵–۱۸۵۲٫ [Google Scholar] [CrossRef] [PubMed]
- Zare, Y. 3Recycled Polymers: Properties and Applications. Available online: https://www.smithersrapra.com/SmithersRapra/media/Sample-Chapters/Recycled-Polymers-Properties-and-Applications,-Volume-2.pdf (accessed on 28 November 2017).
- Gallop, W.A.; Evans, M.G.; Mithal, A.K. Knife Having Superior Functionality and Appeal. U.S. Patent 12/455,322, 3 December 2009. [Google Scholar]
- Achilias, D.; Giannoulis, A.; Papageorgiou, G. Recycling of polymers from plastic packaging materials using the dissolution–reprecipitation technique. Polym. Bull. ۲۰۰۹, ۶۳, ۴۴۹–۴۶۵٫ [Google Scholar] [CrossRef]
- Plastic Coding Guidelines in the United States. Available online: http://www.natureworksllc.com/~/media/The_Ingeo_Journey/EndofLife_Options/plastic_codes/2008_04_10_plastic_code_guidelines_pdf.pdf (accessed on 6 November 2017).
- Recycled Plastics in Food Packaging. Available online: https://www.fda.gov/food/ingredientspackaginglabeling/packagingfcs/recycledplastics/default.htm(accessed on 25 June 2017).
- Franz, R.; Welle, F. 23—Recycling packaging materials A2—Ahvenainen, Raija. In Novel Food Packaging Techniques; Woodhead Publishing: Cambridge, UK, 2003; pp. 497–۵۱۸٫ [Google Scholar]
- Aizawa, H.; Yoshida, H.; Sakai, S.-I. Current results and future perspectives for Japanese recycling of home electrical appliances. Resour. Conserv. Recycl.2008, ۵۲, ۱۳۹۹–۱۴۱۰٫ [Google Scholar] [CrossRef]
- Shih, L.-H. Reverse logistics system planning for recycling electrical appliances and computers in Taiwan. Resour. Conserv. Recycl. ۲۰۰۱, ۳۲, ۵۵–۷۲٫ [Google Scholar] [CrossRef]
- Park, C.-H.; Jeon, H.-S.; Yu, H.-S.; Han, O.-H.; Park, J.-K. Application of Electrostatic Separation to the Recycling of Plastic Wastes: Separation of PVC, PET, and ABS. Environ. Sci. Technol. ۲۰۰۸, ۴۲, ۲۴۹–۲۵۵٫ [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, H.; Guo, J.; Xu, Z.; Zhou, Y. Recycle technology for recovering resources and products from waste printed circuit boards. Environ. Sci. Technol. ۲۰۰۷, ۴۱, ۱۹۹۵–۲۰۰۰٫ [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Hewage, K. How “green” are the green roofs? Lifecycle analysis of green roof materials. Build. Environ. ۲۰۱۲, ۴۸, ۵۷–۶۵٫ [Google Scholar] [CrossRef]
- PlasticsEurope. Available online: https://committee.iso.org/files/live/sites/tc61/files/The%20Plastic%20Industry%20Berlin%20Aug%202016%20-%20Copy.pdf(accessed on 21 July 2017).
- Rudolph, N.; Kiesel, R.; Aumnate, C. Understanding Plastics Recycling: Economic, Ecological, and Technical Aspects of Plastic Waste Handling; Carl Hanser Verlag GmbH Co. KG: Munich, Germany, 2017. [Google Scholar]
- Bates, M.P. Plastics Recycling: The Big Challenges, the Big Opportunities UK and International. Presented at Recoup Annual Conference, Peterborough, UK, 29 September 2016. [Google Scholar]
© ۲۰۱۷ by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
